Page 8 - hypertension_newsletter (5)
P. 8
REFLECTIONS rtensio n
Hypertension Global Newsletter #2 Hype
Hype
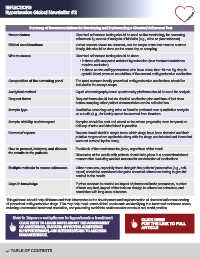
Summary of Recommendations for Developing and Implementing a Chemical Adherence Test
n oisnetr
Nomenclature Chemical adherence testing should be used as the terminology for assessing
adherence by means of analysis of biofluids (e.g., urine or plasma/serum).
Ethical considerations Verbal consent should be obtained, and the scope of the test must be outlined.
Ideally, this should be done on the same day as sampling.
Who to screen Chemical adherence testing should be done:
• In those with suspected resistant hypertension (true treatment resistance
requires exclusion)
• In those on two antihypertensives who have a less than 10 mm Hg drop in
systolic blood pressure on addition of the second antihypertensive medication
Composition of the screening panel The most common locally prescribed antihypertensive medications should be
included in the assays scope.
Analytical method Liquid chromatography-mass spectrometry platforms should be used for analysis.
Request forms Request forms should include detailed medication plan and time of last dose
before sampling; other patient characteristics can be collected too.
Sample type Qualitative screening using urine or blood is preferred over quantitative analysis
as cut-offs (e.g., for Cmin) cannot be sourced from literature.
Sample stability and transport Samples should be sent and stored on ice where pragmatic; room temperature
delivery of urine and dried blood is possible.
Format of reports Reports should detail in simple terms which drugs have been detected and their
putative ranges where applicable along with the drugs not detected and those that
were not covered by the assay.
How to present, interpret, and discuss Feedback of the result must be given, regardless of the result.
the results to the patients
Discussion of the results with patients should take place in a nonconfrontational
manner after excluding medical reasons for nondetection of medications.
Multiple methods to assess adherence Other measures, especially those that gain the patients’ perspective (e.g., self-
report) should be considered alongside chemical adherence testing to give full
context to the results.
Gaps in knowledge Further research is needed on impact of pharmacokinetic parameters, number
of tests required, impact of the tests on change in adherence behaviour, and
correlation with long-term outcomes.
This guidance should help clinicians and their laboratories in the development and implementation of chemical adherence testing
of prescribed antihypertensive drugs. This may help meet unmet clinical needs such as identifying true treatment resistance cases,
reducing unwarranted treatment escalation, and preventing avoidable cardiovascular events in real world practice.
How to improve compliance to hypertension treatment CLICK HERE
FOR THE LINK TO FULL
CLICK HERE TO LEARN MORE ABOUT THE ASSESSMENT ARTICLE
OF ADHERENCE, FACTORS AFFECTING ADHERENCE
IN HYPERTENSION, AND INTERVENTIONS TO ADDRESS
NONADHERENCE.
TABLE OF CONTENTS